Welcome to Digital Drug Property Database (DDPD)
A comprehensive open resource for providing manually curated and standardized digital properties of drugs to support drug-likeness evaluation and drug discovery.
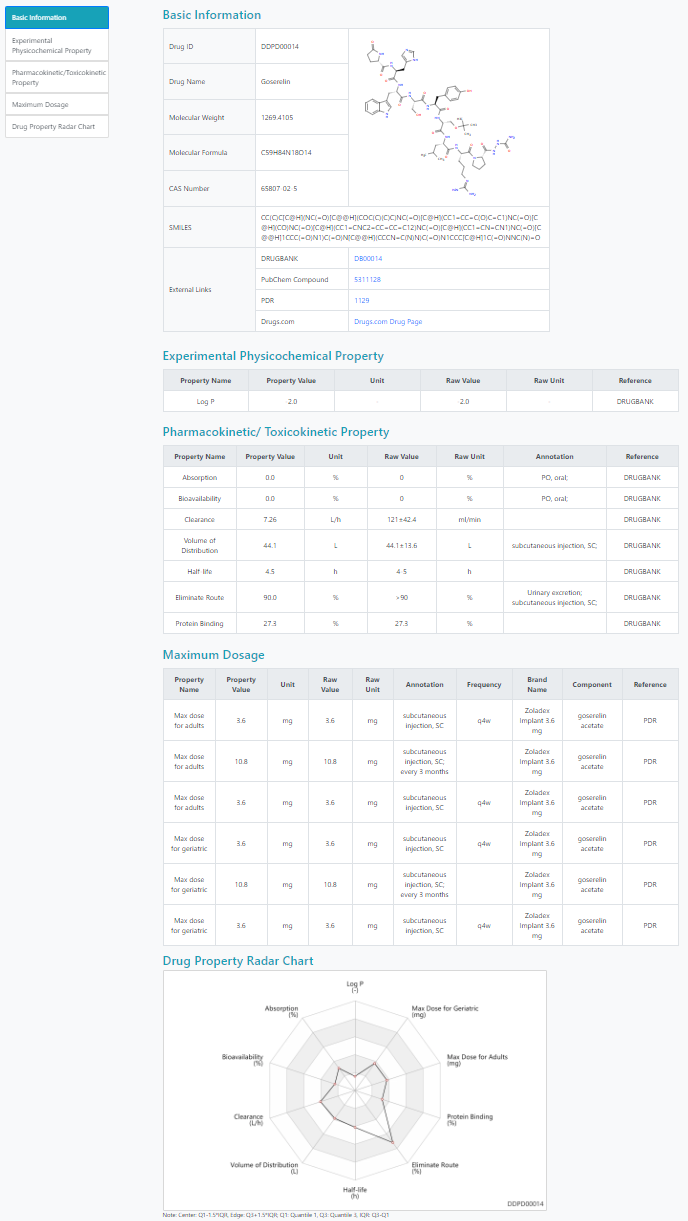
To function as an effective drug, quantitative principles (e.g Lipinsiki’s Rule of Five) of molecular properties including the physicochemical and pharmacokinetics properties have to be obeyed so that the molecules can reach the drug target in sufficient concentration for enough time. Drug-likeness, a vital evaluation for compounds selection in early phase of drug discovery, is typically modeled by physicochemical and pharmacokinetic properties. Here, we establish The Digital Drug Property Database (DDPD), a structured digital database providing manually assembled and standardized drug property data of approved drugs. The overall aim is to provide high quality comprehensive resource of drug-likeness related properties including experimental physicochemical properties, pharmacokinetic/toxicokinetic properties and maximum dosages of approved drugs. Curated from literature and databases including DRUGBANK, T3DB, ATSDR and PDR, the assembled digital drug-likeness related drug property data is believed to be a useful resource for driving drug-likeness evaluation and prediction potentially with state-of-the-art Machine Learning based multivariate technologies and is hoped to become a hub for in silico modeling of drug-likeness to support drug discovery.
[05/01/2021] DDPD version 1.0 is fully ready for service.
Li Q, Ma SY, Zhang XL, Zhai ZY, Zhou L, Tao HD, Wang YC, Pan JB*. DDPD 1.0: a manually curated and
standardized
database of digital properties of approved drugs for drug-likeness evaluation and drug development. Database
(Oxford). 2022 Feb 9; 2022: baab083.
DOI: 10.1093/database/baab083.
PMID: 35139189.
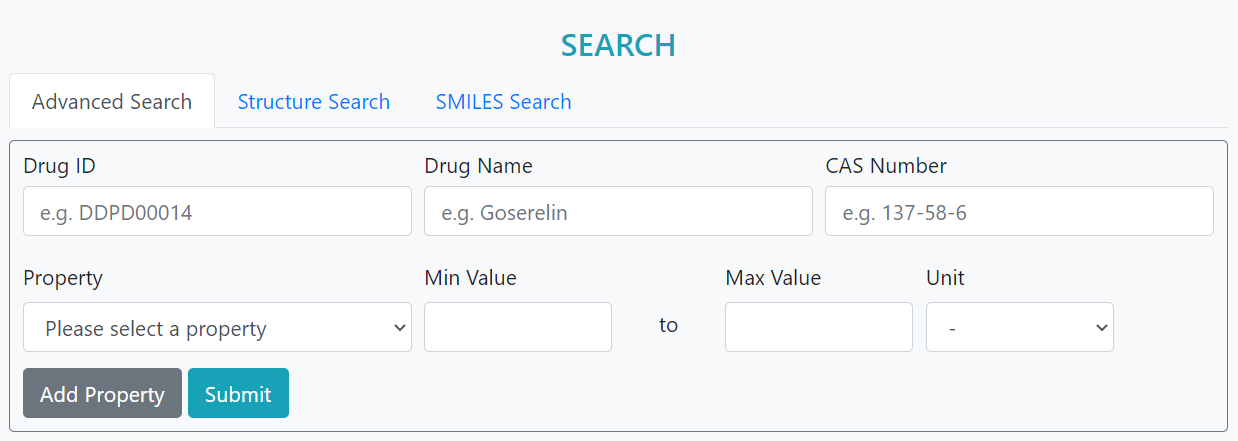
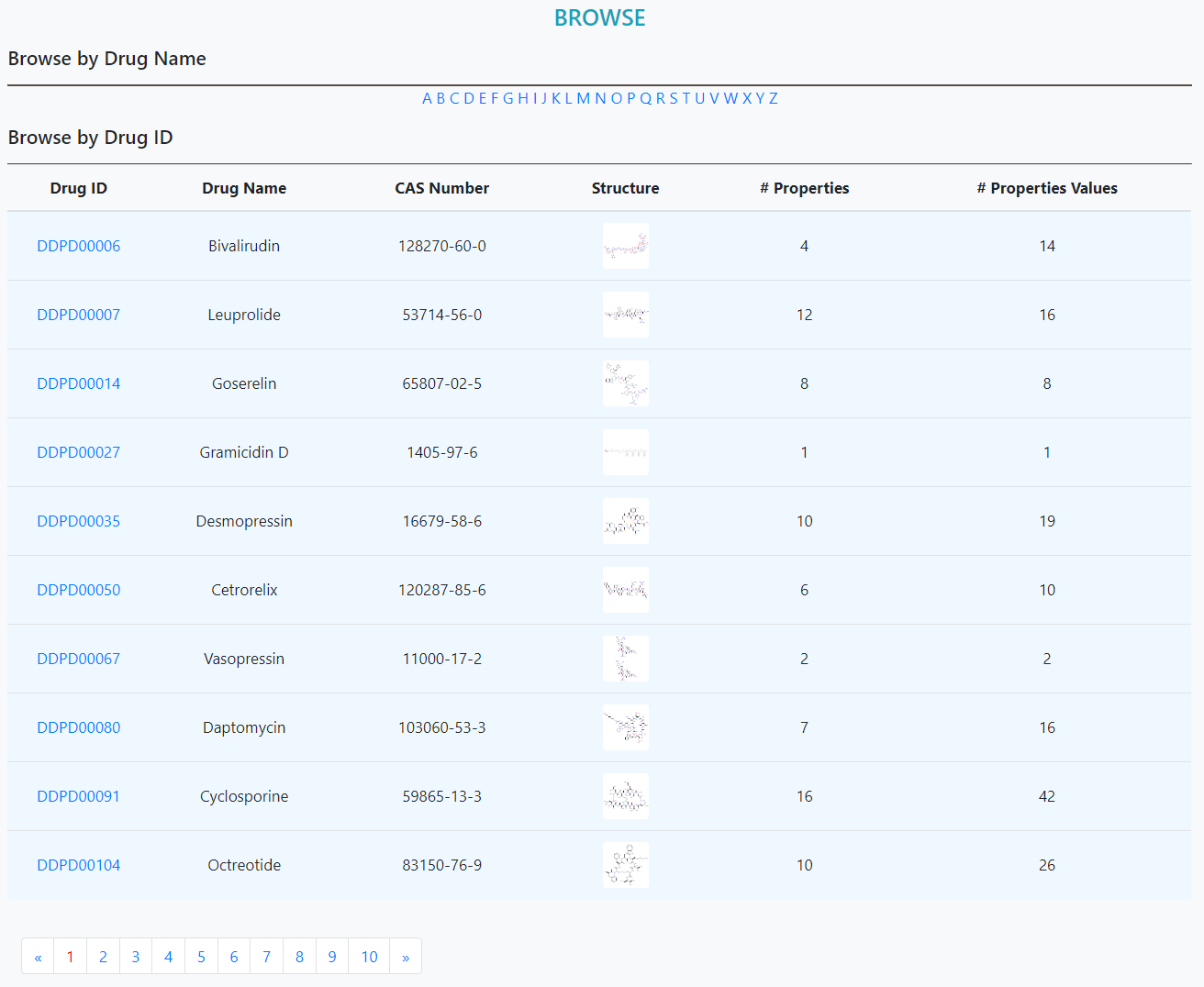
> Search property values by drug name, structure or "SMILES" string.
> Search drugs with self-defined property values.
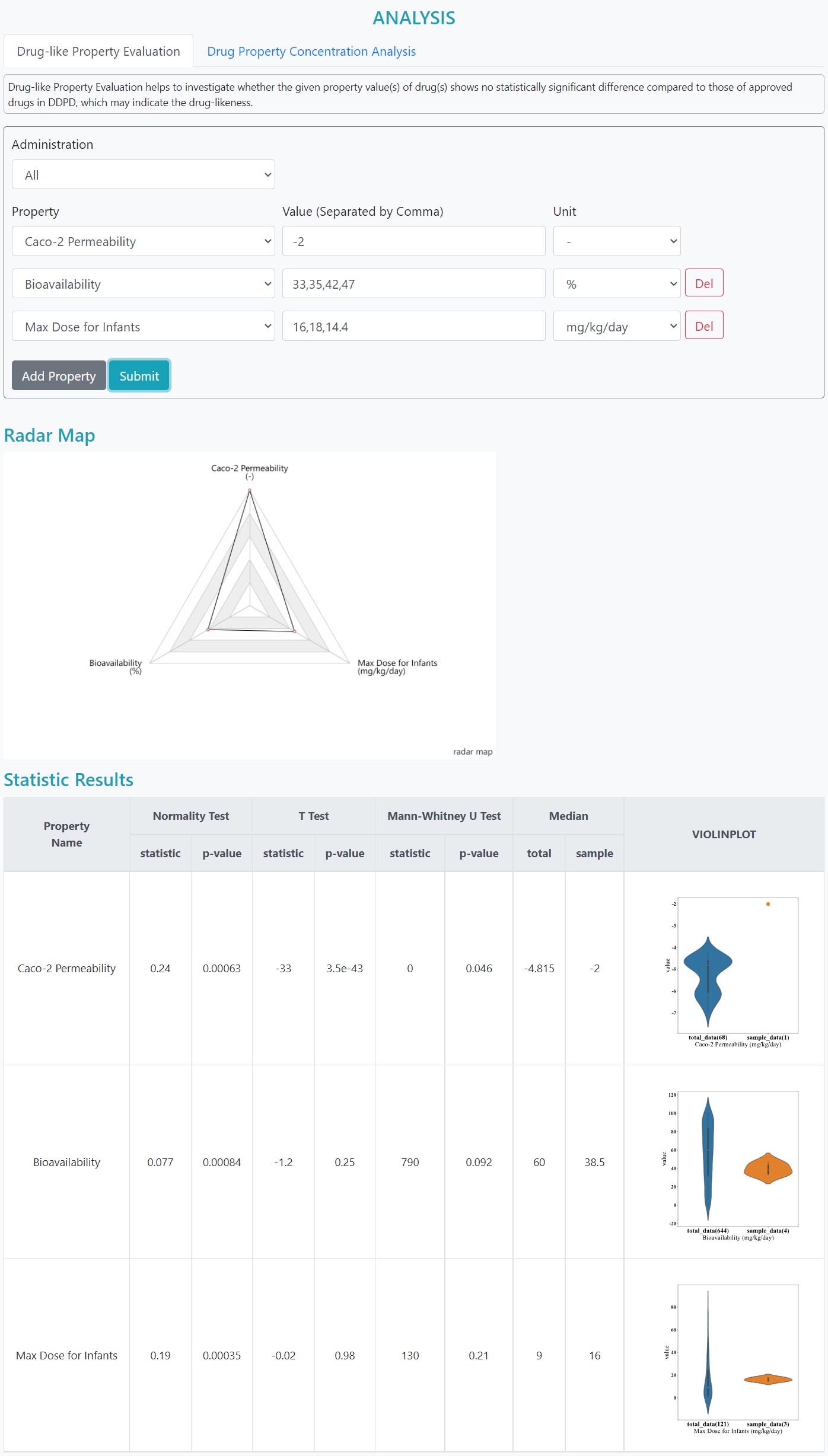
> Compare property values user inputs with those of approved drugs.
> Perform property concentration analysis on drug set.
> Drug-likeness evaluation
> Development of Drug-likeness Rules
> Drug property prediction with machine learning methods
> Pharmacokinetic/Toxicokinetic/Dosage analysis of a drug set
> Drug screening and drug design
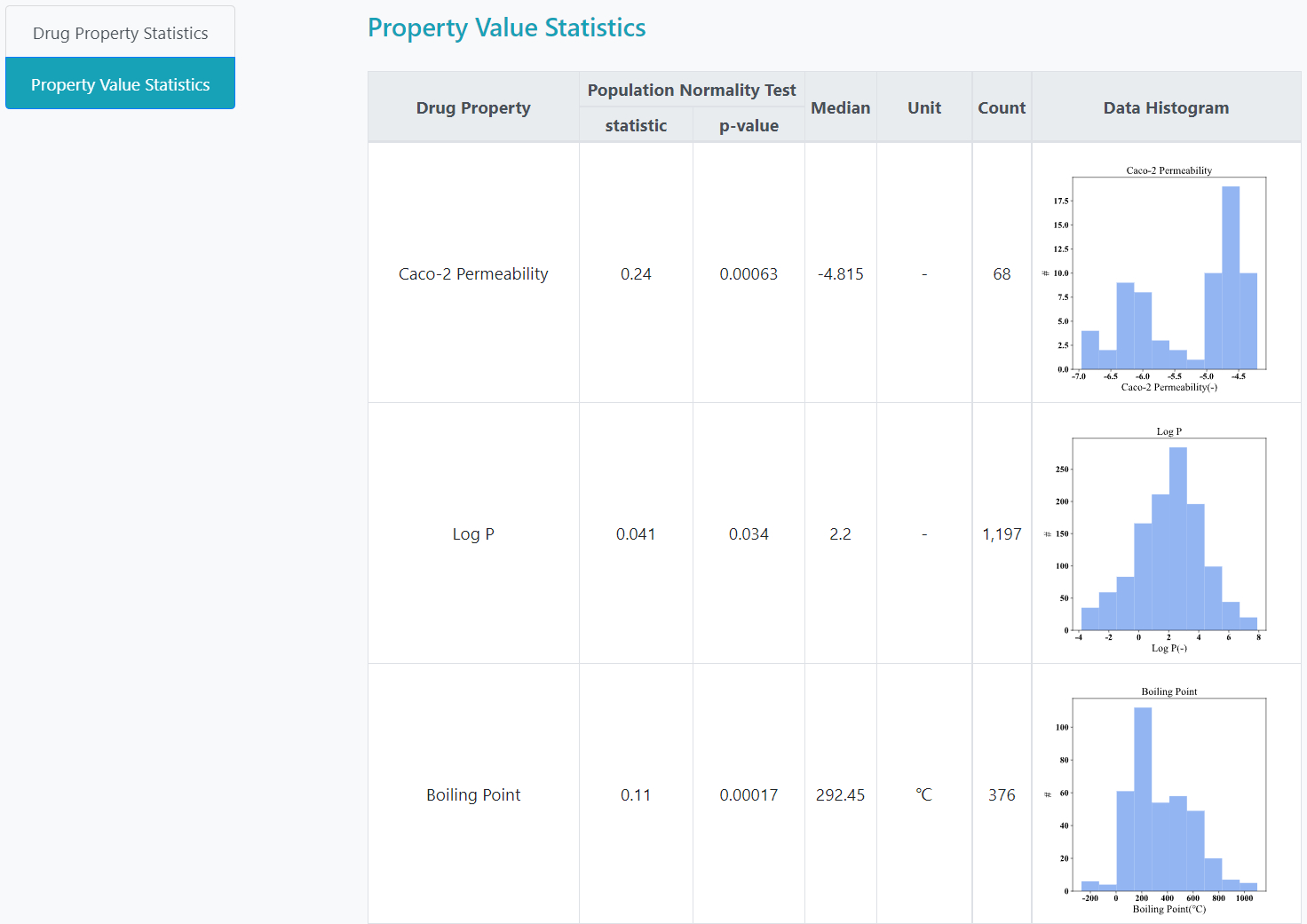
 Database Statistics
Database Statistics
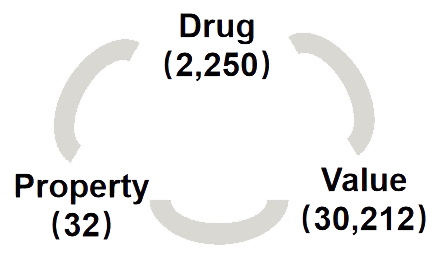
> Total approved drugs: 2,250
> Total drug properties: 32
> Total non-redundant drug-property pairs: 18,011
> Total drug property values: 30,212
| Experimental Physicochemical Property | ||
|---|---|---|
| Property | # Drugs | # Values |
| Melting Points | 1,308 | 1,312 |
| Log P | 1,232 | 1,232 |
| Water Solubility | 765 | 772 |
| pKa | 466 | 495 |
| Boiling Point | 389 | 392 |
| Log S | 162 | 162 |
| Caco-2 Permeability | 78 | 78 |
| Total | 4,400 | 4,443 |
| Maximum Dosage | ||
|---|---|---|
| Property | # Drugs | # Values |
| Max Dose for Children | 569 | 1,848 |
| Max Dose for Adults | 925 | 1,708 |
| Max Dose for Adolescents | 601 | 1,441 |
| Max Dose for Geriatric | 728 | 1,444 |
| Max Dose for Infants | 265 | 635 |
| Max Dose for Elderly | 225 | 352 |
| Max Dose for Neonates | 151 | 325 |
| Total | 3,464 | 7,753 |
| Pharmacokinetic/Toxicokinetic Property | ||
|---|---|---|
| Property | # Drugs | # Values |
| Half-life | 1,697 | 3,485 |
| Clearance | 1,147 | 2,298 |
| Volume of Distribution | 1,185 | 2,048 |
| Eliminate Route | 964 | 1,932 |
| Toxicity LD50 | 780 | 1,765 |
| Protein Binding | 1,180 | 1,553 |
| T Max | 808 | 1,346 |
| Bioavailability | 644 | 961 |
| Metabolic | 292 | 401 |
| Absorption | 362 | 398 |
| AUC | 246 | 372 |
| C Max | 591 | 1,083 |
| Tss | 90 | 98 |
| Toxicity MRLs | 33 | 83 |
| Toxicity TDLo | 44 | 71 |
| Toxicity Lethal Dose | 40 | 65 |
| Toxicity LDLo | 21 | 29 |
| Css | 23 | 28 |
| Total | 10,147 | 18,016 |
To get a better experience with this site, Chrome,Firefox, Microsoft Edge are advised!
RELATED RESOURCE:  |
|
 |
|
 |
|